in vivo-jetPEI® is a ready-to-use cationic polymer reagent recommended for in vivo transfection of DNA, siRNA, miRNA, shRNA and other oligonucleotide...

RNA/DNA Therapeutics
New range of proprietary cationic lipids for LNP formulation
Efficient
Modulate LNP properties to adapt the biodistribution depending on the therapeutic purpose
Secure
Use a unique lipid structure protected by an independent patent owned by Polyplus®
Accelerate your project
in vitro and in vivo proof of concept have been successfully performed
Flexible
Comprehensive library enabling efficient screening and refinement to identify the perfect LipidBrick® for each therapeutic application
DNA and RNA therapeutics offer promising new treatments for a wide range of diseases, and lipid nanoparticles (LNPs) are an essential tool in their delivery. Those treatments include both prophylactic vaccines that prevent infection by triggering the patient’s immune system and therapeutic vaccines/drugs that will be used to cure a patient from a specific disease. As the properties of LNPs influence their delivery efficacy, their stability as well as their biodistribution, each therapy will require its own LNP formulation depending on the genetic material, application, and tissue(s)/organ(s) of interest. Thus, to tailor the delivery system to the needs, a wide variety of lipids modulating the physico-chemical properties of LNP is required to ensure therapeutic success.
As an innovator in the field of nucleic acid delivery, Polyplus® has developed a new range of cationic lipids, named LipidBrick® dedicated to the formulation of lipid nanoparticles (LNPs). These active lipids protect the mRNA molecules and play an important role in the transfection capacity of LNPs. Importantly, by being based on an imidazolium polar head, LipidBrick® broadens the spectrum of current LNP applications in terms of potency and biodistribution by adding an overall positive charge to LNPs: this translates into greater delivery of mRNA to the lungs and/or the spleen while reducing accumulation in the liver compared to LNPs based on ionizable lipids.
Polyplus’ goal is to support customers from R&D to commercialization. Within the LipidBrick® library, LipidBrick® IM21.7c is the cationic lipid (active lipid) used in the formulation of jetMESSENGER® and in vivo–jetRNA®+ , allowing a seamless transition between our ready-to-use reagents and your LNP formulation finet-uned to your specific needs and applications.
Moreover, LipidBrick® is based on a unique lipid structure protected by an independent patent owned by Polyplus®.
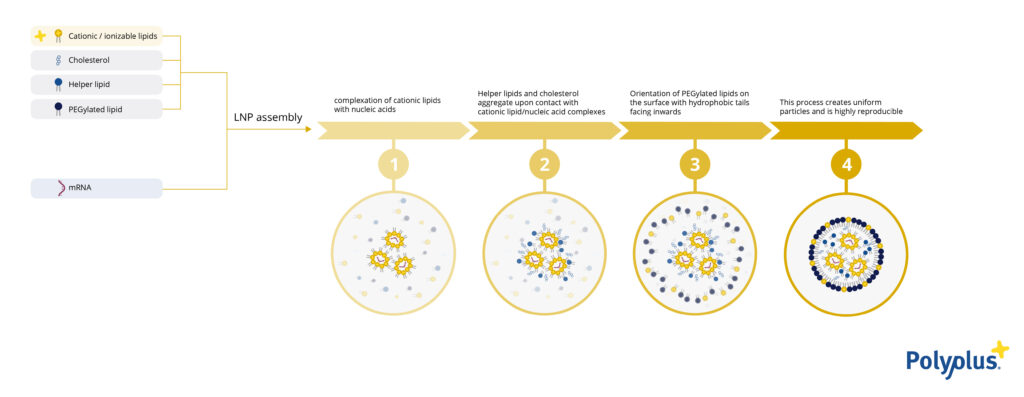
Figure: LNP-mRNA formulation using a microfluidic system.
Table: LipidBrick® Library description
| LipidBrick® name | Linear Formula | Molecular Weight (g/mol) | IUPAC name |
| IM21.7c | C59H117ClN2 | 890.03 | 3-butyl-1-(2,6-dimethyl-14-octadecyldotriacontan-9-yl)-1H-imidazol-3-ium chloride |
| IM3c | C41H79ClN2 | 635.55 | (Z)-1-(heptatriacont-9-en-19-yl)-3-methyl-1H-imidazol-3-ium chloride |
| IM12c | C48H95ClN2 | 735.75 | 1-(2,6-dimethyl-14-tetradecyloctacosan-9-yl)-3-methyl-1H-imidazol-3-ium chloride |
| IM13c | C64H127ClN2 | 960.18 | 3-methyl-1-(24-octadecyldotetracontan-19-yl)-1H-imidazol-3-ium chloride |
| IM15c | C53H97ClN2 | 797.82 | 3-methyl-1-(7-octadecyl-1-phenylpentacosan-2-yl)-1H-imidazol-3-ium chloride |
| IM16c | C56H111ClN2 | 847.97 | 1-(2,6-dimethyl-14-octadecyldotriacontan-9-yl)-3-methyl-1H-imidazol-3-ium chloride |
| IM20c | C59H115ClN2 | 888.03 | (Z)-3-butyl-1-(24-tetradecyloctatriacont-9-en-19-yl)-1H-imidazol-3-ium |
| IM22c | C51H101ClN2 | 777.83 | 3-butyl-1-(2,6-dimethyl-14-tetradecyloctacosan-9-yl)-1H-imidazol-3-ium chloride |
| IM23c | C43H85ClN2 | 665.62 | 3-butyl-1-(14-(3,7-dimethyloctyl)-2,6,17,21-tetramethyldocosan-9-yl)-1H-imidazol-3-ium chloride |
| IM25c | C57H113ClN2O | 877.99 | 3-(2-hydroxyethyl)-1-(24-tetradecyloctatriacontan-19-yl)-1H-imidazol-3-ium chloride |

These FAQs are organized by application to guide you to find the best answer possible.

You have access to all the documents related to the transfection reagent.

Search for publications in our Transfection Database with Polyplus transfection reagents

This lexicon will help you to understand the different terms related to Polyplus-transfection®.