Summary
Setting up robust and reproducible bioprocesses is a top priority for all biotechnology companies focused on developing life changing AAV-based gene therapies. To do so, innovators crave for breakthrough technologies to maximize productivity while controlling the cost of goods (CoGs) of their AAV vector manufacturing workflow, key to accelerate the speed to commercialization of their clinical programs pipeline. Helper-free triple plasmid transfection of HEK293 cells – adherent or suspension – is the most used upstream method for AAV manufacture. Observing consistent plasmid DNA delivery among multiple production runs is critical but requires some effort as finding optimal transfection conditions for a given bioprocess is not plug and play. Still, significant advances have been recently made towards standardization of the triple transfection process. Here, we discuss how optimizing the transfection step during upstream process development leverages tremendous benefits on overall costs, robustness and scalability of an AAV production platform.
 |
About the AuthorGuillaume Freund, Ph.D. is our Scientific Support Manager at Polyplus-transfection®. After graduating from the Heriot-Watt University of Edinburgh with a Bachelor of Science, he completed a Master’s degree followed by a PhD in Biotechnology at the University of Strasbourg in the field of antibody engineering. He joined Polyplus-transfection® in September 2015. |
Manufacturing rAAV through transient plasmid DNA delivery
Gene Therapy innovators must deal with constant pressure to get their product(s) to the clinic as fast as possible. In this aspect, the speed and flexibility of transient transfection of plasmid DNA to generate functional recombinant Adeno-Associated Virus (AAV) particles are cutting edge advantages over other techniques such as stable cell line generation. Despite being widely used, triple transfection has often been overlooked and labelled as “challenging”. While timing is key in an ultra-competitive market, a growing number of companies realize the importance of establishing robust and highly optimized manufacturing bioprocesses to produce enough potent viral particles. Approximatively 1:100,000 assembled AAV capsids will display the desired clinical output in humans1 emphasizing the fact that viral manufacturers must invest resources to build efficient AAV vector production platforms. And this goal can only be reached by having a robust and consistent plasmid DNA delivery method in place in the upstream process. Allocating proper resources to fine-tune triple transfection can have a huge positive outcome on the amount of functional viral particles produced.
One protocol will never rule them all
The path to successfully reach clinical and commercial stages largely depends on the Process Development (PD) efforts deployed for AAV manufacturing, whether you outsource them to a Contract Development and Manufacturing Organization (CDMO) or perform them in-house. The triple aim of such PD activities consists in:
- Achieving highest viral titer…
- With highest viral particle functionality…
- Using an economically viable manufacturing process.
To reach this simply put yet ambitious goal, innovators need to develop a consistent and reproducible process. Plasmid DNA delivery is at the core of success since setting up a robust triple transfection step is often considered as the main challenge of upstream bioprocessing. Thus, it is critical to determine the best set of conditions for each specific AAV manufacturing workflow. There are many factors impacting the output of plasmid DNA delivery experiments to produce AAV vectors (Figure 1).
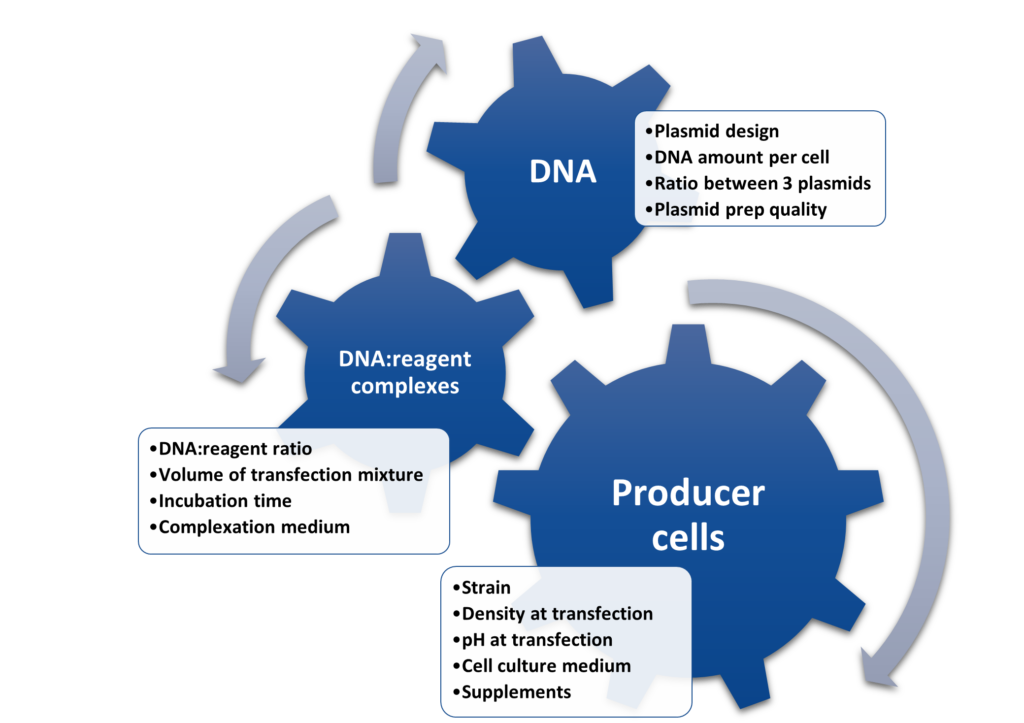
Figure 1: non-exhaustive set of parameters to explore during optimization of a triple transfection process for AAV manufacturing.
Each variable should be carefully studied during PD to identify the best performing transfection conditions and fine-tune the design space to operate in to target appropriate Critical Quality Attributes (CQA) of the process. Until now, most of the work done to improve rAAV production in the field often relied on optimizing a single factor at a time. However, this approach is no longer favored because of (1) the multiplicity of factors contributing to rAAV generation, and (2) how these variables can interact. Design of Experiments (DOE) is now widely used to tackle this complexity by enabling to optimize each factor and ultimately determine a unique set of parameters for each specific viral vector manufacturing process. DOE approach allows to screen and optimize many factors, but you first need to identify and rank your drivers to draw clear experiments objectives as optimal transfection parameters cannot be determined in one single experiment.
Also, best performing transfection conditions for one serotype are not necessarily optimal for another – even when producing two different vectors using an identical tissue culture system. This really underlines that spending resources in early stage to tweak the workflow is key. Sometimes less is more. For example, when using the appropriate transfection reagent and conditions, reducing the total DNA amount can improve cell viability and increase overall rAAV particles quality for optimal potency in vivo without compromising on productivity.
rAAV particle potency: an overlooked driver during upstream PD efforts?
Appropriate outputs are required to characterize any manufacturing process such as the physical titer expressed as Viral Genomes (VG) per mL of crude harvest. This readout is universally used for rAAV vector titration. VG copies are measured by quantitative PCR (qPCR) or droplet digital PCR (ddPCR), both techniques allowing to screen and select best performing experimental conditions in a sensible timeframe. While relatively quick and easy to determine, the physical titer is not always directly correlated with the final potency and quality of the recombinant vector produced. Thus, even if reaching the highest physical titer remains of primary importance when conducting upstream PD activities, the number of VG produced should not be used as sole criterion to cement best performing transfection conditions moving forward. Assessing functionality of produced viral particles is critical to align both UpStream Processes (USP) and DownStream Processes (DSP) objectives when developing a novel rAAV production platform. In this way, quantifying the proportion of full particles in the crude harvest while measuring their infectivity is required to confirm that the rAAV production method can consistently produce enough potent vector. Infectivity or functionality is usually determined with an in vitro assay using the closest cell type possible to the one targeted in the clinical application2. Thereby focusing on VG titer alone might lead to back-and-forth optimization between USP and DSP because of poor quality viral particles. You cannot spread yourself too thin when you want to quickly get your product into the clinic. Each PD experiment matters, we will never say it enough: transfection conditions and cultivation parameters need to be optimized for each unique rAAV vector and cell culture setup. And Polyplus offers everything needed to do so.
Premium reagents and support from the non-viral gene delivery pioneer
For many years now, PEIpro® is the leading DNA transfection reagent used in viral vector manufacturing processes for Gene & Cell Therapy products. PEIpro® has proven its efficiency, robustness, and scalability in large-scale manufacturing of preclinical and clinical batches of rAAV. Abeona Therapeutics recently exemplified these characteristics by demonstrating the scalability of their transfection-based production process from 50 L to 500 L stirred tank bioreactor3. However, 500 L scale is just the beginning. To keep increasing productivity while easing the scale-up of bioprocesses to even larger bioreactors, Polyplus launched the new generation reagent FectoVIR®-AAV several months ago to revolutionize transfection-based rAAV manufacturing platforms: more titer, more flexibility, more scalability, more of everything can be expected when using FectoVIR®-AAV for your rAAV transfection process in suspension cells. Optimizing transfection parameters with FectoVIR®-AAV based on a DOE approach can lead to unmatched yield improvements compared to standard triple transfection experiments using commercially available PEI. By combining its proprietary plasmid technology to FectoVIR®-AAV reagent in a rational DOE approach, the clinical-stage genetic medicine company LogicBio Therapeutics demonstrated more than 10-fold increased titers in HEK293 suspension cells4.
Polyplus does not only provide gold standard transfection reagents such as FectoVIR®-AAV at different quality grades from RUO to GMP (Read more on The importance of cGMP compliant raw materials in viral vector manufacturing) Technical expertise is also delivered through the Scientific Support department committed to assist our customers and partners, from early PD efforts up to scaling-up and industrialization of viral vector manufacturing process. The team is composed of highly qualified research scientists dedicated to understanding specific needs, providing highest level of scientific advice and offering the best solution above and beyond transfection s. All of this to ensure that each scientist successfully optimizes their viral vector production process to ultimately bring Gene and Cell Therapies to market faster.
Conclusion
Polyplus’ unique transfection technologies supports continuous PD efforts deployed by viral vector innovators to make AAV manufacture affordable. Superior reagents such as FectoVIR®-AAV combined to DOE is most probably the best approach to optimize triple transfection experiments and in fine unlock the potential of each specific viral vector manufacturing process. It is also essential to keep in mind that rAAV producing factories are human cultured cells. In that sense, identifying the best set of parameters to cultivate and transfect them in appropriate conditions in a well-characterized process is key to ultimately reach maximal productivity.
Sources
- Hitchcock T, et al. Development Approaches to Adenoassociated Virus Production. BioProcess International (2017).
- Navigating the Complexities of AAV Scale-Up and Manufacturing. Genetic Engineering & Biotechnology News (2020).
- Sanderson TP, et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell & Gene Therapy Insights (2021).
- Hebben M & Nyamay’Antu A. Optimizing the AAV Transfection Process in Suspension Cells. BioProcess International (2021).

