FectoPRO is a transfection reagent specifically optimized for transient protein and antibody expression in CHO and HEK293 cells.

We use cookies to help you navigate efficiently and perform certain functions. You will find detailed information about all cookies under each consent category below.
The cookies that are categorized as "Necessary" are stored on your browser as they are essential for enabling the basic functionalities of the site. ...
Necessary cookies are required to enable the basic features of this site, such as providing secure log-in or adjusting your consent preferences. These cookies do not store any personally identifiable data.
Functional cookies help perform certain functionalities like sharing the content of the website on social media platforms, collecting feedback, and other third-party features.
Analytical cookies are used to understand how visitors interact with the website. These cookies help provide information on metrics such as the number of visitors, bounce rate, traffic source, etc.
Advertisement cookies are used to provide visitors with customized advertisements based on the pages you visited previously and to analyze the effectiveness of the ad campaigns.
To achieve successfully simultaneous or expression of several genes into one plasmid, there are several genetic engineering strategies including using multicistronic plasmids and multi-cassette plasmids.
 |
Alengo Nyamay’antu, Ph.D, Scientific Marketing & Communication Team leader at Polyplus®. |
Multricistronic plasmids enable simultaneous expression levels of two or more genes, as expression is driven by a single promoter. They rely on the use of linker sequences, mainly IRES (Internal ribosome entry sites) elements or 2A peptides to add several Open Reading Frames (ORF) under the control of one promoter. These linker sequences, IRES and 2A peptides, respectively act as additional ribosome recruitment sites to allow start of transcription for the next ORF, and as as self-cleaving peptides that will self-cut within the single transcript from the promoter to generate in both cases an individually expressed protein from each ORF.
When the aim is to achieve similar co-expression levels, multicistronic plasmids are no longer the go to solution. The preferred solution is to have distinct promoters for each gene of interest, because with multicistronic plasmids, the gene of interest furthest to the promoter shows a decreased expression level compared to the gene closest to the promoter. An additional advantage of having distinct promoter for each gene of interest is in being able to restrict expression, for example to a subcellular compartment.
Classically, a strategy can be to co-deliver several plasmids, each containing the promoter and the gene of interest. However, in this case simultaneous expression in one single cell is not guaranteed. Therefore, one single plasmid that can contain several cassettes is the best fitting solution.
To engineer a single plasmid with several cassettes, the traditional cloning method is based on the use of predefined standard plasmid backbones designed for co-expression of only 2 genes of interests. However, these dual promoter plasmids are not meant for co-expression of 3-4 genes, unless additional cloning steps are done to reengineer these plasmids that can become time-consuming, expensive and even inefficient depending on the complexity of the plasmid DNA construct desired. Therefore, we developed an innovative genetic engineering assembly technology e-Zyvec that makes multi-cassette plasmids easy to make, irrespective of the number of genes of interests needed, and that with complete freedom of design.
Here is an example of a multiple gene expression plasmid designed from scratch. The aim was to demonstrate co-expression of four proteins, each under a specific promoter to drive distinct subcellular expression to the golgi apparatus, mitochondria, nucleus and to the plasma membrane.
Complete freedom of design enabled us to:
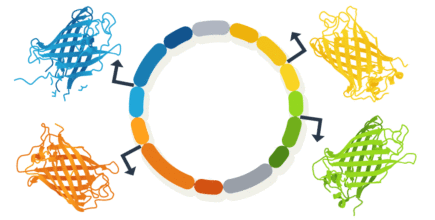
Figure 1. Design of multi-expression plasmid to ensure simulteanous co-expression of four proteins.
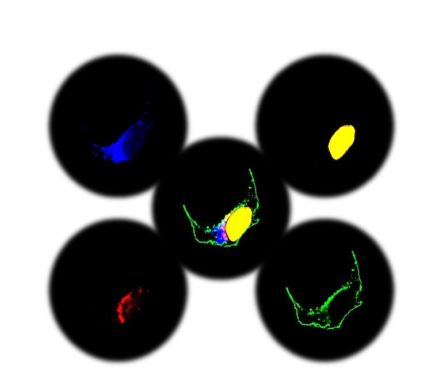
Figure 2. Multi-expression plasmid ensured specific subcellular expression of each gene of interest to the golgi apparatus (blue), mitochondria (red), nucleus (yellow) and to the plasma membrane (green).
We offer a plasmid engineering service that is based on our assembly technology e-Zyvec® and dedicated software which makes it possible to design and produce any complex plasmid within a few weeks.
Follow these 3 steps, to receive your ready-to-use plasmid DNA at your bench!