jetMESSENGER® is a designed for high mRNA transfection efficiency in primary cells, cancer cell lines, neurons, and stem cells.
Authors: Karine Le Blay, Sylvie Remaud, Alengo Nyamay’Antu, Barbara Demeneix, Géraldine Guérin-Peyrou, Maxime Dumont, Patrick Erbacher.
Introduction
In light of their remarkable properties of both self-renewal and differentiation, adult neuronal stem cells are a unique cellular system that has demonstrated promising potential in developmental biology, regenerative medicine and disease therapy. Cultivating adult neuronal stem cells in vitro as non-adherent free-floating spheroids is a frequently used 3D cell culture model to mimic the in vivo niche. Growing neural stem cells as neurospheres are an established approach to study molecular mechanisms that drive self-renewal and differentiation. Gene expression strategies to examine the role of proteins in modulating these cellular mechanisms rely on the ability to induce ectopic or endogenous gene overexpression.
As neurosphere are composed of both stem cells and progenitor cells, it is important to find a gene delivery method that will target all cell types, irrespective of their self-renewal capacity and consequently of their division rate. As an alternative to viral-based transfection methods which could give rise to undesirable random genomic integration and subsequent potential pathogenicity, Polyplus-transfection® has developed an mRNA transfection reagent jetMESSENGER® for difficult to transfect cells. Messenger RNA transfection is a growing alternative transfection method to plasmid DNA delivery which allows reproducible and high transfection efficiencies in a wide variety of cells by circumventing the need for nuclear import of DNA, hindered in slow to none dividing cells (1). This process has many advantages over DNA transfection, including high percentage of transfected cells irrespective of their division rate, earlier and more controlled protein expression with no risk of genome integration. Concomitantly, toxicity and immune response usually induced by mRNA entry into the cytosol has been reduced with the latest generation of modified mRNA (2,3).
Here, we propose a mRNA transfection based-method to reach high transfection efficiencies in neuronal stem cells grown in spheroids, based on our combined expertise on neuronal stem cells and the novel mRNA transfection reagent jetMESSENGER® developed by Polyplus-transfection. The aim of this study was two-fold. Firstly, to test mRNA as an alternative method to DNA transfection for neuronal stem cells. Secondly, to determine the transfection efficiency of the heterogeneous population of neurosphere-forming stem cells including neuroblasts, oligodendrocytes progenitor cells, as well as other cell types such as astrocytes and glial cells (Fig 1.).
Fig 1. Comparison between mRNA transfection using jetMESSENGER® and DNA transfection using jetPRIME®.
Material & Methods
Neurospheres were transfected with either EGFP mRNA using jetMESSENGER® (Polyplus-transfection) or Lipofectamine®-2000 (Thermo Fisher Scientific®), or with DNA using jetPRIME® (Polyplus-transfection®) according to the manufacturer’s recommendations. Neurospheres were gently dissociated and plated together at equal densities of 40 000 cells per well of a 24 well-plate in complete medium. Twenty four hours post-seeding of cells, transfection was performed by adding 0.5 µg of mRNA. The GFP encoding mRNA was used with respectively a mRNA: reagent ratio of 1:2.4 and 1:3 ratio for jetMESSENGER® and Lipofectamine®. For DNA transfection, 0.5 µg of EGFP plasmid DNA was complexed with 1.5 µL of jetPRIME® per well. EGFP expression in transfected cells was examined by Fluorescence microscopy from 24 hours and up to 7 days post-transfection.
Results
First, we analyzed the transfection efficiency of both jetMESSENGER® and Lipofectamine®-2000 transfection reagents. To this end, transfected cells were fixed at different time points following transfection (from 24 hours to 7 days). For both transfection reagents, we detected EGFP expression 24 hours post-transfection. Both continued to provide high levels of EGFP expression up to 7 days post-transfection (Fig 2A).
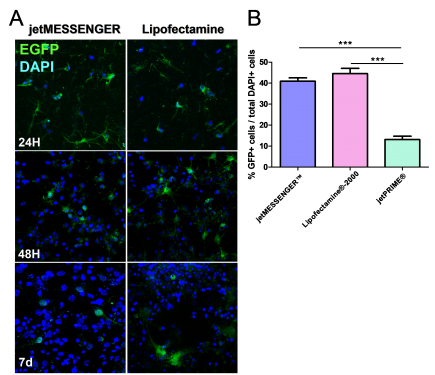
Fig 2. Comparative transfection efficiency of mRNA versus DNA transfection reagent
To evaluate more precisely the transfection efficiency, we quantified the percentage of EGFP+ transfected cells 48 hours post-transfection. We observed a 4-fold increase in transfected cells using either jetMESSENGER® (40.93 ± 1.57) or Lipofectamine®-2000 (44.56 ± 2.51) compared to jetPRIME® (13.15 ± 1.59) (Fig 2B), showing that mRNA transfection is more efficient for transfection of differentiated neuronal cells from neurospheres than DNA transfection.
Since neurospheres contain a mix of cell types, we studied which cell type is preferentially targeted by each transfection reagents. To this end, immunohistochemistry using antibodies directed against markers expressed in NSCs (GFAP and SOX2) or astrocytes (GFAP) was performed. For each reagent, the EGFP signal was mostly detected in the cytoplasm of (i) NSC co-expressing SOX2 and GFAP and (ii) SOX2+ progenitors (Fig 3A).
jetMESSENGER® transfects more efficiently both NSCs and progenitors (53.37 ± 2.55 and 23.04 ± 2.81) compared to either Lipofectamine®-2000 (42.55 ± 2.32 and 13.44 ± 2.09) or jetPRIME® (39.42 ± 4.73 and 18.53 ± 3.67). Moreover, jetMESSENGER® privileges transfection of NSCs and progenitors as opposed to astrocytes, GFAP+ astrocytes showing only 16.92 ± 1.9 transfection efficiency as opposed to either Lipofectamine® 2000 (29.46 ± 2.2) or jetPRIME® (28.42 ± 4.49) (Fig 3B). Thus, jetMESSENGER® shows more specific targeting of NSC/progenitors than Lipofectamine® 2000.
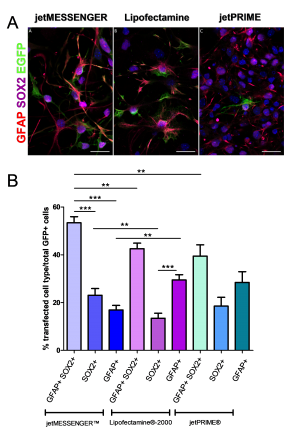
Fig 3: mRNA transfection using jetMESSENGER® targets preferentially both NSCs – and progenitors-derived neurospheres.
Conclusion & perspectives
Here we show that mRNA transfection is an efficient technique to express exogenous proteins in neurospheres. Indeed, mRNA transfection leads to significantly higher transfection efficiency than DNA transfection, by a 3-fold increase.
To target different cell types within the neurosphere, such as neuronal stem cells and progenitors, mRNA delivery using jetMESSENGER® was the most efficient approach.
In the future, we will use a mRNA delivery approach for our gene expression experiments in Neurospheres using jetMESSENGER® as it is the most efficient way to target our cells of interest, Neuronal Stem Cells and progenitors.
References
(1) Yamamoto, A., et al (2009) Eur J Pharm Biopharm. 71(3):484-9.
(2) Kariko et al, Mol Ther., 2008.
(3) Uchida et al, Pharmaceutics, 2015.